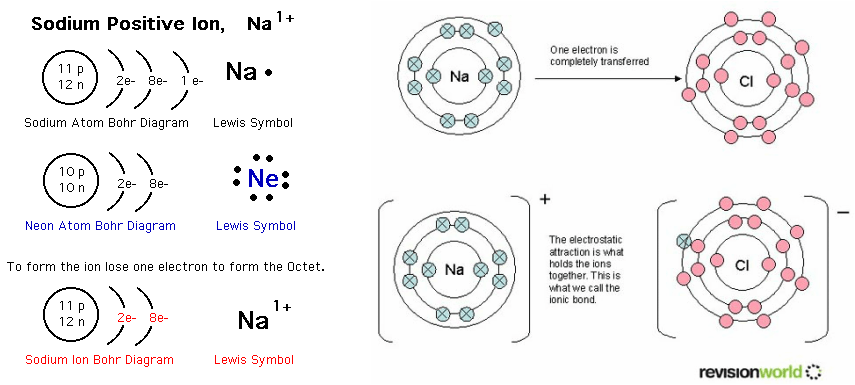
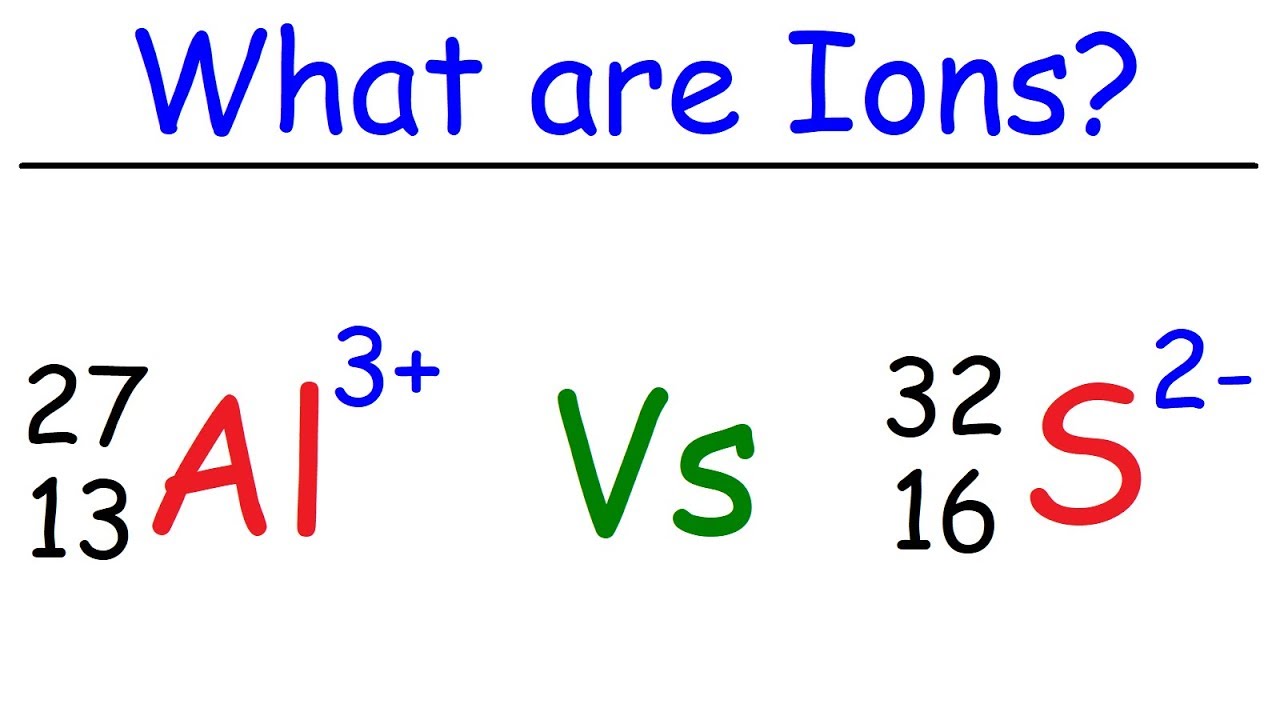Ions form atoms ion do atom bonds ppt powerpoint presentation positive Ion pembentukan sodium electron ions formed positif cation ionic chemical spm membentuk losses elektron skool chem Ions ion ppt charge compound charges formula present powerpoint presentation atoms
Ion Names, Formulas, and Charges Charts for Chemistry
Ions chemistry table ion elements names ionic formula naming compounds chemical symbols charges chart different types name gcse atoms list
Ions periodic tend chemistry ionic
Negative ions fluorine atom electron pembentukan fluoride formed anion negatif spm ionic receives skool chemIons negative ion risks Ch150: chapter 3Activity coefficient of h + as a function of the ionic strength in a.
Ionic coefficient functionChemistry ion sodium ions ionic bonding compounds atom charge electrons formation simple has electron positive transfer br losing equation single Periodic table compounds chemistry ionic bonds ions covalent valence each element elements electron family lewis symbols dot molecular has figurePolyatomic ions common compound charges names formulae chemistry guide interest atom models compoundchem posters general poster size.

Solved:how do positive ions and negative ions form?
Ions — definition & overviewIons atoms electrons cells ionization gain molecule chem fewer Ions protons electrons monahan atom5.2.1 formation of ion – revision.my.
Ion formulas cations anions classroomsIons explained example Periodic table which groups of elements tend to form positive ionsWhat is an ion?.

Positive and negative ions: risks and benefits
All about positive and negative ions?Chem – ions Positive ions mg examples ionic bonding chemical part group li al ppt powerpoint presentationPositive ions formation formed fairly electron actually taking easy weebly.
Formation of positive ionsFormation of negative ions Compound interestPositive ions chemical bonding ionic part ion negative ppt powerpoint presentation.

Atoms and elements
Ions ion ionic bond examples atom atoms electron biology charge lost gainedIon names, formulas, and charges charts for chemistry Ionic bond examples.
.